Overview

The Cozzolino group addresses research challenges related to current problems of technological and environmental significance. Central to our efforts is the concept of engineered complexity: the rational combination of well defined components to give functional smart molecules and materials. There currently three trends: a) light-enhanced reactivity of transition-metal complexes using photoswitchable ligands, b) molecular recognition driven self-assembly through pnictogen bonding, and c) p-block element catalysts supported in metal-organic frameworks. Students in my group acquire a broad spectrum of practical skills including inorganic synthesis and reactivity, structure determination through spectroscopic and diffraction techniques, and modern computational methods.
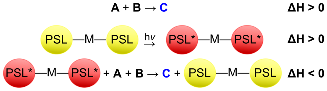
Redox-active unsaturated early transition metal complexes are capable of activating small molecule substrates. The resulting species are often thermodynamically stable products that only participate in further reactivity when mediated by exotic reagents. We are using photoswitchable ligands (PSLs) to modulate the redox properties of coordination complexes. These smart molecules will be used to drive thermodynamically uphill transformations of ubiquitous small molecules into value-added products.
Smart coordination complexes for small molecule activation

Secondary bonding interactions (SBIs) are structure directing attractive interactions involving heavy main-group atoms. We are preparing a series building blocks that are anticipated to self-assemble through SBIs. Our elements of choice are antimony and bismuth (in the +3 oxidation state). We use density functional theory to aid in programming predictable self-assembly into the molecules. To date we have made columnar structures, self-assembled inverted bi-layers and even an inorganic analogue of the guanine-cytosine base pair. We have also designed a series of antimony containing molecules that can bind anions. We are exploring these as a novel class of catalyst.
Molecular recognition and self-assembly with heavy p-block elements

P-block elements have many exciting catalytic applications, but they often sit in the shadow of their neighbors on the periodic table. They, however, have unique geometries from d-block elements and readily engage in two electron redox processes. The d-block elements that do undergo 2 electron redox processes tend to be rare and expensive. One of the issues that often plagues p-block elements in catalytic processes is deactivation through aggregation, or disproportionate. We use metal-organic frameworks to site-isolate such species in an effort to prevent these undesirable side reactions. We design our MOFs to have binding sites for the p-block elements that mimic homogeneous catalysts. In doing so we strive to maintain the activity while enhancing the stability and allowing for catalysts recovery and reuse. Our current focuses are on Al, Pb and I catalysts.
Metal organic frameworks as supports for p-block catalysts